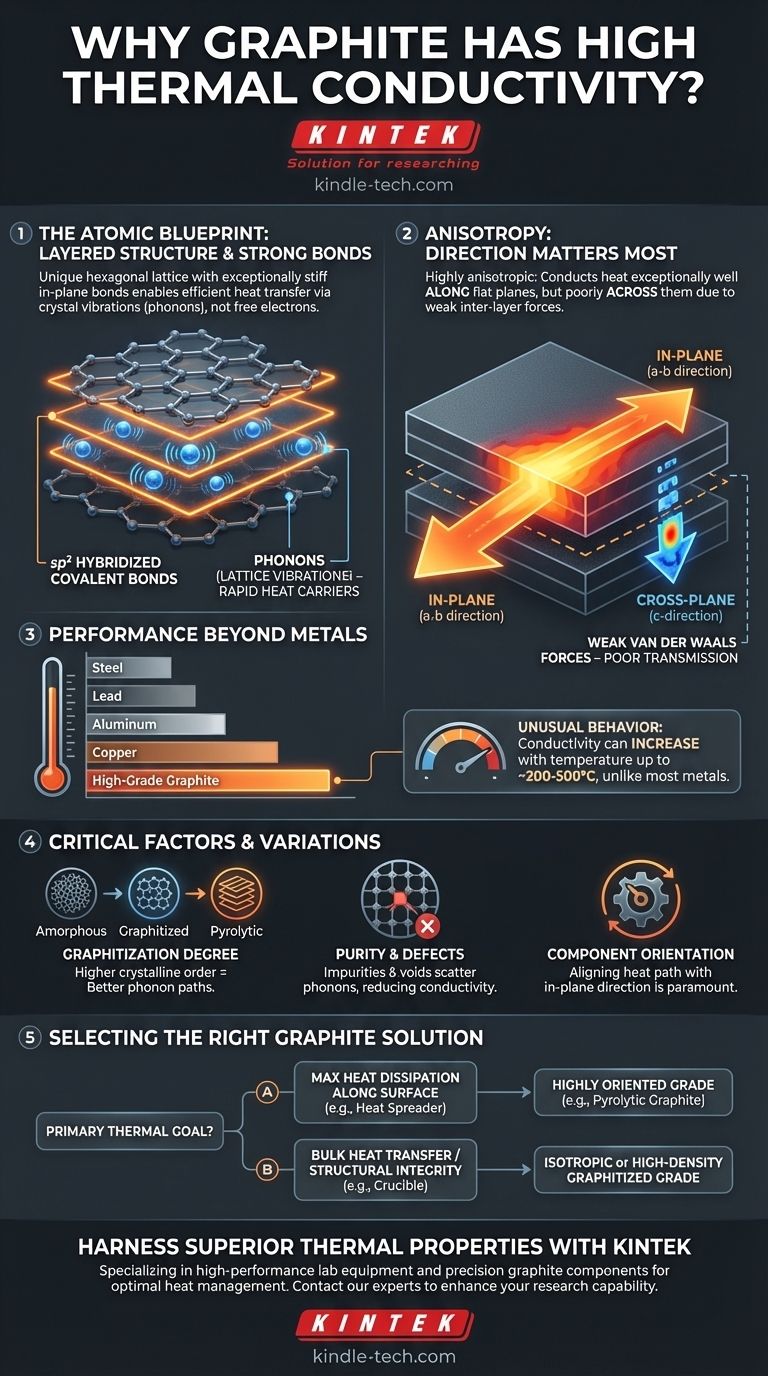
本質的に、黒鉛の高い熱伝導率は、そのユニークな層状原子構造に由来します。これらの層内の強い共有結合により、格子振動の形で熱エネルギーが、ピンと張った太鼓の皮を伝わる音のように、例外的な速度と最小限の抵抗で伝達されます。
重要なのは、黒鉛が均一に導電性を持つわけではないことを理解することです。これは高度に異方性のある材料であり、平坦な面沿いでは熱を非常にうまく伝導しますが、その面を横切る方向では伝導性が低いことを意味します。この方向性こそが、その実用上の応用において最も重要な要素です。
熱伝達のための原子設計図
黒鉛が鉄や鉛を含む多くの金属を上回る理由は、金属における自由電子によるものではなく、結晶格子内の物理的な振動の効率によるものです。
sp²混成軌道の役割
黒鉛層内の各炭素原子は、六角形の格子内で他の3つの炭素原子と結合しています。これらはsp²混成軌道結合であり、グラフェンのような他の炭素同素体に見られるのと同じ強力な結合タイプです。これらの結合は信じられないほど硬く強く、剛直で平坦なシートを形成します。
熱キャリアとしての格子振動(フォノン)
黒鉛のような非金属固体では、熱は主にフォノン、すなわち振動エネルギーの量子化されたパケットによって伝達されます。鐘をたたくことを想像してください。聞こえる音は、振動として材料を伝わるエネルギーです。
黒鉛格子の1つの部分が加熱されると、その原子はより激しく振動します。面内結合が非常に強く、構造が非常に整然としているため、これらの振動はエネルギー損失をほとんど伴わずに隣接する原子へ効率的に伝達されます。
異方性:二つの方向の物語
黒鉛の特性の秘密は、その二つの明確な構造的特徴にあります。
- 面内(a-b方向): 平らな六角形の層は、極めて高い熱伝導率を持ちます。熱はこの面に沿って急速に伝わります。
- 層間(c方向): 層自体は積み重ねられており、非常に弱いファンデルワールス力によって結合されています。これらの弱い結合は振動の伝達が苦手であるため、層間での熱伝導率は著しく低くなります。
この差は劇的であり、面内熱伝導率は層間熱伝導率の数百倍になることがあります。
他の材料との性能比較
黒鉛の熱性能は、一般的に導電性が高いと見なされる金属と比較すると、直感に反することがよくあります。
一般的な金属を上回る
指摘されているように、特定の黒鉛グレードの熱伝導率は、鉄、鋼、鉛よりも優れています。高品質の黒鉛は、特に重量あたりの比較では銅やアルミニウムの導電率に匹敵することもあり、軽量な熱管理において優れた選択肢となります。その電気伝導率も高く、熱性能と相関していることがよくあります。
温度要因
一般的に熱伝導率が温度上昇とともに低下する金属とは異なり、多くのグレードの黒鉛は特異な挙動を示します。その熱伝導率は、ある点(通常は200~500°C付近)まで温度とともに上昇することがあり、その後低下し始めます。これにより、金属の効果が薄れる高温用途において、黒鉛は非常に有用になります。
トレードオフとバリエーションの理解
黒鉛の選択は、万能の解決策ではありません。その有効性は、材料のグレードと最終用途での配向に完全に依存します。
異方性がもたらす重大な影響
最も一般的な間違いは、黒鉛の方向性伝導性を考慮しないことです。コンポーネントが黒鉛層を横切る方向(c方向)に熱が流れるように設計されている場合、期待される性能は劇的に低下します。適切な配向が最も重要です。
すべての黒鉛が同じではない
「黒鉛」という言葉は、幅広い材料を指します。
- 無定形炭素: 熱伝導率が非常に低い無秩序な構造。
- 黒鉛化炭素: 2500°C以上の非常に高い温度で熱処理され、より秩序だった結晶構造を作り出した材料。黒鉛化の度合いが高いほど、熱伝導率は高くなります。
- 熱分解黒鉛: 非常に秩序だった形態で、極端な異方性を持ち、利用可能な最高の面内熱伝導率の一部を提供します。
純度と欠陥の役割
結晶格子内の不純物、空隙、欠陥は、フォノンが移動するためのクリーンな経路を妨げます。これらは熱流を妨げる「散乱サイト」として機能します。したがって、高品位の合成黒鉛に見られるような、より純粋で完全な結晶構造を持つものの方が、常に優れた熱伝導率を持ちます。
用途に合わせた適切な選択
正しい黒鉛グレードと配向を選択することが成功の鍵となります。あなたの決定は、解決すべき主要な熱的課題によって導かれるべきです。
- 表面に沿った最大の放熱(例:ヒートスプレッダ)が主な焦点の場合: 熱流の望ましい方向に材料の面が揃うように、熱分解黒鉛のような高度に配向されたグレードを使用します。
- 複数の方向へのバルク熱伝達(例:るつぼ)が主な焦点の場合: すべての方向でより均一な特性を持つ等方性黒鉛、または金属含浸複合材グレードの方が良い選択肢となる可能性があります。
- 優れた熱管理を伴う高温での構造的完全性が主な焦点の場合: 高純度、高密度の黒鉛化グレードは、機械的強度と熱伝導率のバランスを提供します。
黒鉛の原子構造と熱特性との関連性を理解することで、エンジニアリング目標を達成するための正確な材料を選択できます。
要約表:
| 特性 | 面内(a-b方向) | 層間(c方向) |
|---|---|---|
| 結合の種類 | 強力なsp²共有結合 | 弱いファンデルワールス力 |
| 熱伝導率 | 極めて高い | 著しく低い |
| 主要な熱キャリア | フォノン(格子振動) | フォノン(非効率的に伝達される) |
研究室で黒鉛の優れた熱特性を活用する準備はできましたか?
KINTEKでは、最適な熱管理のために設計された精密黒鉛部品を含む、高性能な実験用機器や消耗品の提供を専門としています。ヒートスプレッダ、るつぼ、またはカスタムの高温ソリューションが必要な場合でも、当社の専門知識により、最大の効率を得るために適切なグレードと配向を入手できます。
KINTEKをイノベーションのパートナーにしましょう。 当社の専門家に今すぐお問い合わせいただき、当社の黒鉛ソリューションが研究室の能力をどのように向上させ、研究を前進させることができるかをご相談ください。
ビジュアルガイド
関連製品
よくある質問
- スパッタリングプロセス中にアルゴンを使用する目的は何ですか?効率的な薄膜堆積を可能にする
- 撹拌速度は銀ナノワイヤーの形態にどのように影響しますか?高純度合成のための撹拌マスター
- スパッタリングの長所と短所は何ですか?優れた薄膜品質と多様性を実現
- 研究室におけるインキュベーターの一般的な用途は何ですか?正確な分析のための微生物培養
- アルゴンガスは何に使われますか?溶接、照明などのための不活性ガスの力を解き放つ
- 嫌気性スラッジの熱衝撃処理に高精度加熱システムが必要なのはなぜですか? バイオ水素収率の最大化
- QPVAナノファイバー膜用の実験用乾燥オーブンの機能は何ですか?精度による構造安定性の達成
- 焼結は多孔性を増加させますか?より強度の高い材料を得るために多孔性を制御する方法



















